Abstract
Background: As new therapeutics for multiple myeloma (MM) are approved in earlier lines of therapy, drug classes with demonstrated benefit may be exhausted after initial therapy, including proteasome inhibitors (PI), immunomodulatory imides (IMiD), and CD38 targeting monoclonal antibodies (MoAB). Clinical recommendations are to utilize unique drug classes at relapse. This study aims to describe relapsed or refractory multiple myeloma treatments and outcomes in clinical trial eligible patients with prior treatment of at least 1 PI, IMiD, and CD38 MoAB and their outcomes (real world overall response rate (rwORR), progression free survival (rwPFS), overall survival (rwOS)) in US community practice.
Methods: This study used Flatiron Health electronic health record (EHR)-derived de-identified database (New York, NY). These data represent ~280 cancer clinics (mostly community-based practices). Inclusion criteria included ≥18 years old, 2+ clinic visits after 2015, measurable disease, prior PI/IMiD/CD38 MoAB exposure, ECOG ≤2, adequate hematologic/renal/hepatic function, and no stem cell transplant within six months of study entry. Study period was treatment initiation at ≥ second line from November 2015 through December 2019, follow-up through December 2020. Patients with multiple eligible lines of therapy, the last eligible line was evaluated. Real world overall response rate was adapted from Foster et al 2019, rwPFS was measured from treatment until death, progression, or start of new line of therapy, and rwOS was measured from treatment until death.
Results: 120 patients were eligible for this study. Median time from diagnosis to study entry was 3.8 years. Half were 70 years or older (n=62, 52%), with 20% (n=24) ISS III, 35% (n=42) high-risk cytogenetics, and 64% (n=77) at ≥5L treatment. At study start, 38% (n=46) had a prior transplant, 73% (n=88) were triple-class refractory, and 21% (n=25) penta-refractory. The most common regimens were either daratumumab-based (n=35), carfilzomib-based (n=25), or elotuzumab-based (n=15). The most frequent regimens were daratumumab/pomalidomide/dexamethasone (n=8), carfilzomib/cyclophosphamide/dexamethasone (n=7), carfilzomib/pomalidomide/dexamethasone (n=7), carfilzomib/dexamethasone (n=5), elotuzumab/lenalidomide/dexamethasone (n=5), and elotuzumab/pomalidomide/dexamethasone (n=5).
The rwORR in this population was 18.33% (95% CI: 11.41-25.26, n=22). The rwORR was lower in key subgroups: younger age (<65 years old: 13.79% [95% CI: 1.24-26.34], n=4/29), high risk cytogenetics (0%, n=0/10), ISS III (12.12% [95% CI: 0.99-23.26], n=4/33), triple-class refractory (15.91% [95% CI: 8.27-23.55], n=14/88), and penta-refractory (4.00% [95% CI: 0-11.68], n=1/25).
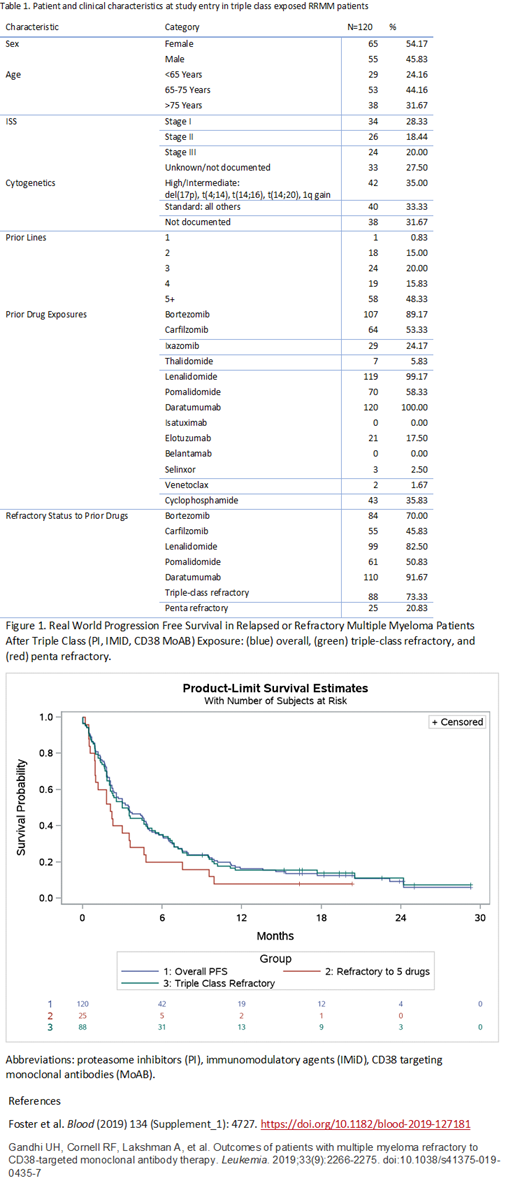
The median rwPFS in this population was 3.5 months (95% CI: 2.3-4.8). The rwPFS were shorter in key subgroups: younger age (<65 years old: 2.1 months [95% CI: 1.8-3.5]), high risk cytogenetics (2.0 months, [95% CI: 0.7-5.0), ISS III (2.2 months [95% CI: 1.6-6.5]), triple-class refractory (3.2 months [95% CI: 2.1-4.8]), and penta-refractory (2.1 [95% CI: 0.9-3.6]).
The median rwOS in this population was 15.8 months (95% CI: 9.9-26.0). The OS was shorter in key subgroups: younger age (10.8 months [95% CI: 4.9-.]), high risk cytogenetics (9.4 months [95% CI: 2.2-27.9]), ISS III (14.6 months [95% CI: 6.1-27.9]), triple class refractory (15.1 months [95% CI: 7.5-25.5]), and penta refractory (7.1 months [95% CI: 3.6-26.0]).
Discussion: In this study of patients that were majority triple-class refractory (PI, IMiD, CD38 MoAbB), low rwORR and short rwPFS were observed. Most patients received re-treatment with at least one drug they had previously failed or were refractory to. Additionally, many patients had additional therapies including novel agents and combinations that could be effective at prolonging OS despite short rwPFS. Compared to academic center patients (Gandhi et al 2019), survival was longer (mOS 15.8 months versus 9.3 months), but in Gandhi 2019, the median time to study entry was 4.5 versus 3.8 years, patients were more penta-refractory (26%) and had prior transplant (72%). Patients who were penta-refractory (bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab) had particularly dismal outcome. Overall, these data suggest need for continued development of effective novel classes of therapies for late line myeloma patients.
Kim: Amgen: Current Employment, Current equity holder in publicly-traded company. Braunlin: Amgen: Current Employment, Current equity holder in publicly-traded company. Mehta: Amgen: Current Employment. Payne: Amgen: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal